Humid Air Properties¶
If you are feeling impatient, jump to Sample HAPropsSI Code, or to go to the code documentation CoolProp.HumidAirProp, otherwise, hang in there.
The equations implemented in CoolProp are based on a publication by Hermann et al. [162], which describes the outcome of the ASHRAE research project ASHREA-RP1485. The same source has been used in the ASHRAE Handbook 2009 to generate reference saturation property tables. The code implemented here passes all tests and reproduces the original data with a very high accuracy. It is applicable for pressure from 0.01 kPa up to 10 MPa, in a temperature range from -143.15 °C up to 350 °C with a humidity ratio from 0 kg of water up to 10 kg of water per kg of dry air.
Humid air can be modeled as a mixture of air and water vapor. In the simplest analysis, water and air are treated as ideal gases but in principle there is interaction between the air and water molecules that must be included through the use of interaction parameters.
Because humid air is a mixture of dry air (treated as a pseudo-pure gas) and water vapor (treated as a real gas), three variables are required to fix the state by the state postulate.
In the analysis that follows, the three parameters that are ultimately needed to calculate everything else are the dry bulb temperature \(T\), the total pressure \(p\), and the molar fraction of water \(\psi_w\). The molar fraction of air is simply \(\psi_a=1-\psi_w\).
Of course, it is not so straightforward to measure the mole fraction of water vapor molecules, so other measures are used. There are three different variables that can be used to obtain the mole fraction of water vapor without resorting to iterative methods.
Humidity ratio
The humidity ratio \(W\) is the ratio of the mass of water vapor to the mass of air in the mixture. Thus the mole fraction of water can be obtained from
or
where the ratio of mole masses \(\varepsilon\) is given by \(\varepsilon=M_w/M_a\)
Relative Humidity
The relative humidity \(\varphi\) is defined as the ratio of the mole fraction of water in the humid air to the saturation mole fraction of water. Because of the presence of air with the water, the pure water saturated vapor pressure \(p_{w,s}\) must be multiplied by an enhancement factor \(f\) that is very close to one near atmospheric conditions.
Mathematically, the result is
where
The product \(p_s\) is defined by \(p_s=fp_{w,s}\), and \(p_{w,s}\) is the saturation pressure of pure water (or ice) at temperature \(T\). This yields the result for \(\psi_w\) of
Dewpoint temperature
The dewpoint temperature is defined as the temperature at which the actual vapor pressure of water is equal to the saturation vapor pressure. At the given dewpoint, the vapor pressure of water is given by
and the mole fraction of water vapor is obtained from
Once the state has been fixed by a set of \(T,p,\psi_w\), any parameter of interest can be calculated
Molar Volume¶
The bracketed term on the right hand side is the compressibility Z factor, equal to 1 for ideal gas, and is a measure of non-ideality of the air. The virial terms are given by
where the virial coefficients are described in ASRAE RP-1485 and their values are provided in Humid Air Validation. All virial terms are functions only of temperature.
Usually the temperature is known, the water mole fraction is calculated, and \(\bar v\) is found using iterative methods, in HAProps, using a secant solver and the first guess that the compressibility factor is 1.0.
Molar Enthalpy¶
The molar enthalpy of humid air is obtained from
with \(\bar h\) in kJ/kmol. For both air and water, the full EOS is used to evaluate the enthalpy
which is in kJ/kmol, using the mixture \(\bar v\) to define the parameter \(\delta=1/(\bar v \bar \rho_c)\) for each fluid, and using the critical molar density for the fluid obtained from \(\bar \rho_c=1000\rho_c/M\) to give units of mol/m3. The offset enthalpies for air and water are given by
respectively. The enthalpy per kg of dry air is given by
Enhancement factor¶
The enhancement factor is a parameter that includes the impact of the air on the saturation pressure of water vapor. It is only a function of temperature and pressure, but it must be iteratively obtained due to the nature of the expression for the enhancement factor.
\(\psi_{w,s}\) is given by \(\psi_{w,s}=fp_{w,s}/p\), where \(f\) can be obtained from
Isothermal Compressibility¶
For water, the isothermal compressibility [in 1/Pa] is evaluated from
with
in kPa/(kg/m3). And for ice,
Sample HAPropsSI Code¶
To use the HAPropsSI function, import it and do some calls, do something like this
#import the things you need
In [1]: from CoolProp.HumidAirProp import HAPropsSI
#Enthalpy (J per kg dry air) as a function of temperature, pressure,
# and relative humidity at dry bulb temperature T of 25C, pressure
# P of one atmosphere, relative humidity R of 50%
In [2]: h = HAPropsSI('H','T',298.15,'P',101325,'R',0.5); print(h)
50423.45039107799
#Temperature of saturated air at the previous enthalpy
In [3]: T = HAPropsSI('T','P',101325,'H',h,'R',1.0); print(T)
290.9620924692057
#Temperature of saturated air - order of inputs doesn't matter
In [4]: T = HAPropsSI('T','H',h,'R',1.0,'P',101325); print(T)
290.9620924692057
Table of Inputs/Outputs to HAPropsSI¶
Parameter |
Units |
Input/Output |
Description |
|---|---|---|---|
|
K |
Input/Output |
Wet-Bulb Temperature |
|
J/kg dry air/K |
Output |
Mixture specific heat per unit dry air |
|
J/kg humid air/K |
Output |
Mixture specific heat per unit humid air |
|
J/kg dry air/K |
Output |
Mixture specific heat at constant volume per unit dry air |
|
J/kg humid air/K |
Output |
Mixture specific heat at constant volume per unit humid air |
|
K |
Input/Output |
Dew-Point Temperature |
|
J/kg dry air |
Input/Output |
Mixture enthalpy per dry air |
|
J/kg humid air |
Input/Output |
Mixture enthalpy per humid air |
|
W/m/K |
Output |
Mixture thermal conductivity |
|
Pa-s |
Output |
Mixture viscosity |
|
mol water/mol humid air |
Input/Output |
Water mole fraction |
|
Pa |
Input |
Pressure |
|
Pa |
Input |
Partial pressure of water vapor |
|
Input/Output |
Relative humidity in [0, 1] |
|
|
J/kg dry air/K |
Input/Output |
Mixture entropy per unit dry air |
|
J/kg humid air/K |
Input/Output |
Mixture entropy per unit humid air |
|
K |
Input/Output |
Dry-Bulb Temperature |
|
m \(^3\) /kg dry air |
Input/Output |
Mixture volume per unit dry air |
|
m \(^3\) /kg humid air |
Input/Output |
Mixture volume per unit humid air |
|
kg water/kg dry air |
Input/Output |
Humidity Ratio |
|
Output |
Compressibility factor (\(Z = pv/(RT)\)) |
Psychrometric Chart¶
(Source code, png, .pdf)
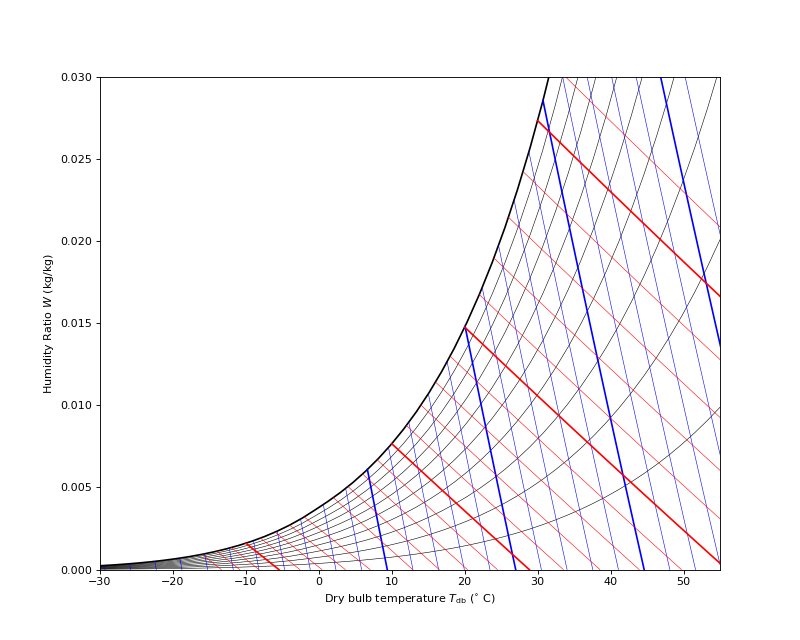
Humid Air Validation¶
Values here are obtained at documentation build-time using the Humid Air Properties module
In [5]: %run 'fluid_properties/Validation/HAValidation.py'
Replicating the tables from ASHRAE RP-1485
A.6.1 Psychrometric Properties of Moist Air at 0C and Below
Saturated air at 101.325 kPa
====================================================
T Ws v h s
C kgw/kg_da m3/kgda kJ/kgda kJ/kgda/K
----------------------------------------------------
-60 0.0000067 0.6027 -60.325 -0.2494
-55 0.0000129 0.6169 -55.280 -0.2260
-50 0.0000243 0.6312 -50.222 -0.2030
-45 0.0000445 0.6454 -45.144 -0.1805
-40 0.0000793 0.6597 -40.031 -0.1583
-35 0.0001379 0.6740 -34.859 -0.1364
-30 0.0002345 0.6883 -29.593 -0.1145
-25 0.0003905 0.7027 -24.181 -0.0924
-20 0.0006373 0.7172 -18.542 -0.0699
-15 0.0010207 0.7319 -12.560 -0.0465
-10 0.0016062 0.7468 -6.070 -0.0215
-5 0.0024863 0.7622 1.165 0.0057
0 0.0037900 0.7780 9.475 0.0364
====================================================
A.6.2 Psychrometric Properties of Moist Air at 0C and Above
Saturated air at 101.325 kPa
====================================================
T Ws v h s
C kgw/kg_da m3/kgda kJ/kgda kJ/kgda/K
----------------------------------------------------
0 0.0037900 0.778 9.47 0.0364
5 0.0054247 0.794 18.64 0.0697
10 0.0076626 0.812 29.35 0.1079
15 0.0106938 0.830 42.12 0.1525
20 0.0147605 0.850 57.56 0.2057
25 0.0201734 0.872 76.50 0.2699
30 0.0273329 0.896 100.01 0.3482
35 0.0367601 0.924 129.46 0.4448
40 0.0491445 0.957 166.69 0.5650
45 0.0654161 0.995 214.17 0.7162
50 0.0868629 1.042 275.35 0.9081
55 0.1153262 1.101 355.15 1.1549
60 0.1535446 1.175 460.89 1.4776
65 0.2057936 1.273 604.00 1.9084
70 0.2791668 1.405 803.48 2.5012
75 0.3863984 1.593 1093.39 3.3518
80 0.5529259 1.881 1541.79 4.6511
85 0.8381052 2.367 2307.52 6.8431
90 1.4202351 3.349 3867.63 11.2559
====================================================
A.8.1 Psychrometric Properties of Moist Air at 101.325 kPa
Dry Bulb temperature of 200C
================================================================
W Twb v h s RH
kgw/kg_da C m3/kgda kJ/kgda kJ/kgda/K %
----------------------------------------------------------------
0.00 45.07 1.341 202.52 0.5558 0.0000
0.05 55.38 1.448 346.49 1.0299 0.4850
0.10 61.85 1.556 490.43 1.4736 0.9028
0.20 69.95 1.771 778.25 2.3337 1.5859
0.30 75.00 1.986 1066.01 3.1752 2.1208
0.40 78.51 2.201 1353.73 4.0059 2.5510
0.50 81.12 2.416 1641.42 4.8295 2.9045
0.60 83.14 2.630 1929.09 5.6479 3.2002
0.70 84.76 2.845 2216.73 6.4624 3.4511
0.80 86.09 3.060 2504.37 7.2737 3.6668
0.90 87.20 3.274 2791.99 8.0824 3.8541
1.00 88.15 3.489 3079.60 8.8891 4.0183
================================================================
A.8.2 Psychrometric Properties of Moist Air at 1000 kPa
Dry Bulb temperature of 200C
================================================================
W Twb v h s RH
kgw/kg_da C m3/kgda kJ/kgda kJ/kgda/K %
----------------------------------------------------------------
0.00 90.47 0.136 201.94 -0.1033 0.0000
0.05 107.30 0.147 345.60 0.3175 4.7863
0.10 117.69 0.158 488.97 0.7078 8.9096
0.20 180.72 0.179 775.07 1.4603 15.6512
0.30 138.66 0.200 1060.53 2.1936 20.9304
0.40 144.29 0.222 1345.53 2.9157 25.1764
0.50 148.49 0.243 1630.17 3.6303 28.6655
0.60 151.76 0.264 1914.54 4.3394 31.5835
0.70 154.39 0.284 2198.70 5.0443 34.0601
0.80 156.56 0.305 2482.69 5.7459 36.1883
0.90 158.37 0.326 2766.53 6.4448 38.0369
1.00 159.92 0.347 3050.26 7.1414 39.6575
================================================================
A.8.3 Psychrometric Properties of Moist Air at 2000 kPa
Dry Bulb temperature of 200C
================================================================
W Twb v h s RH
kgw/kg_da C m3/kgda kJ/kgda kJ/kgda/K %
----------------------------------------------------------------
0.00 105.93 0.068 201.34 -0.3045 0.0000
0.05 125.81 0.074 344.62 0.1000 9.3475
0.10 138.03 0.079 487.33 0.4735 17.4003
0.20 153.19 0.089 771.38 1.1917 30.5666
0.30 162.65 0.100 1054.03 1.8898 40.8768
0.40 169.28 0.110 1335.64 2.5761 49.1691
0.50 174.23 0.120 1616.43 3.2542 55.9833
0.60 178.11 0.130 1896.58 3.9265 61.6822
0.70 181.23 0.140 2176.21 4.5942 66.5189
0.80 183.81 0.150 2455.41 5.2582 70.6753
0.90 185.98 0.160 2734.26 5.9193 74.2855
1.00 187.83 0.169 3012.79 6.5780 77.4505
================================================================
A.8.4 Psychrometric Properties of Moist Air at 5000 kPa
Dry Bulb temperature of 200C
================================================================
W Twb v h s RH
kgw/kg_da C m3/kgda kJ/kgda kJ/kgda/K %
----------------------------------------------------------------
0.00 126.87 0.028 199.72 -0.5738 0.0000
0.05 151.76 0.030 341.85 -0.1918 21.5445
0.10 166.94 0.032 482.37 0.1580 40.1047
0.15 177.63 0.034 621.47 0.4958 56.2606
0.20 185.72 0.036 759.34 0.8257 70.4509
0.25 192.15 0.037 896.09 1.1499 83.0138
0.30 197.42 0.039 1031.82 1.4695 94.2140
================================================================
A.8.5 Psychrometric Properties of Moist Air at 10,000 kPa
Dry Bulb temperature of 200C
================================================================
W Twb v h s RH
kgw/kg_da C m3/kgda kJ/kgda kJ/kgda/K %
----------------------------------------------------------------
0.00 142.19 0.014 197.66 -0.7823 0.0000
0.05 171.31 0.015 337.69 -0.4188 39.4620
0.10 188.92 0.016 473.92 -0.0901 73.4579
================================================================
A.9.1 Psychrometric Properties of Moist Air at 101.325 kPa
Dry Bulb temperature of 320C
================================================================
W Twb v h s RH
kgw/kg_da C m3/kgda kJ/kgda kJ/kgda/K %
----------------------------------------------------------------
0.00 54.90 1.681 326.93 0.7901 0.0000
0.05 62.07 1.816 482.76 1.2864 0.0668
0.10 67.00 1.951 638.59 1.7525 0.1244
0.20 73.54 2.221 950.21 2.6573 0.2185
0.30 77.79 2.491 1261.80 3.5436 0.2922
0.40 80.80 2.761 1573.37 4.4192 0.3515
0.50 83.07 3.030 1884.93 5.2876 0.4002
0.60 84.85 3.300 2196.47 6.1509 0.4409
0.70 86.28 3.570 2508.01 7.0102 0.4755
0.80 87.46 3.840 2819.54 7.8664 0.5052
0.90 88.45 4.109 3131.07 8.7200 0.5310
1.00 89.29 4.379 3442.59 9.5715 0.5536
================================================================
A.9.2 Psychrometric Properties of Moist Air at 1000 kPa
Dry Bulb temperature of 320C
================================================================
W Twb v h s RH
kgw/kg_da C m3/kgda kJ/kgda kJ/kgda/K %
----------------------------------------------------------------
0.00 107.70 0.171 326.80 0.1318 0.0000
0.05 118.99 0.185 482.46 0.5751 0.6594
0.10 126.74 0.198 637.99 0.9880 1.2275
0.20 137.03 0.225 948.77 1.7865 2.1564
0.30 143.73 0.252 1259.26 2.5661 2.8838
0.40 148.52 0.279 1569.56 3.3350 3.4688
0.50 152.14 0.306 1879.70 4.0966 3.9495
0.60 154.99 0.333 2189.73 4.8529 4.3515
0.70 157.29 0.360 2499.68 5.6053 4.6927
0.80 159.19 0.387 2809.55 6.3544 4.9860
0.90 160.79 0.414 3119.37 7.1010 5.2407
1.00 162.16 0.441 3429.15 7.8454 5.4639
================================================================
A.9.3 Psychrometric Properties of Moist Air at 2000 kPa
Dry Bulb temperature of 320C
================================================================
W Twb v h s RH
kgw/kg_da C m3/kgda kJ/kgda kJ/kgda/K %
----------------------------------------------------------------
0.00 126.92 0.086 326.68 -0.0685 0.0000
0.05 140.12 0.093 482.14 0.3587 1.3189
0.10 149.16 0.099 637.35 0.7553 2.4551
0.20 161.20 0.113 947.16 1.5209 4.3128
0.30 169.07 0.126 1256.41 2.2675 5.7675
0.40 174.71 0.140 1565.23 3.0031 6.9375
0.50 178.98 0.153 1873.75 3.7313 7.8990
0.60 182.35 0.166 2182.04 4.4542 8.7031
0.70 185.08 0.179 2490.14 5.1730 9.3855
0.80 187.34 0.192 2798.09 5.8886 9.9719
0.90 189.25 0.206 3105.93 6.6015 10.4813
1.00 190.88 0.219 3413.67 7.3123 10.9279
================================================================
A.9.4 Psychrometric Properties of Moist Air at 5000 kPa
Dry Bulb temperature of 320C
================================================================
W Twb v h s RH
kgw/kg_da C m3/kgda kJ/kgda kJ/kgda/K %
----------------------------------------------------------------
0.00 154.63 0.035 326.46 -0.3351 0.0000
0.05 170.96 0.037 481.31 0.0702 3.2972
0.10 182.17 0.040 635.49 0.4448 6.1377
0.15 190.55 0.043 789.13 0.8084 8.6103
0.20 197.14 0.045 942.31 1.1653 10.7820
0.25 202.51 0.048 1095.11 1.5175 12.7047
0.30 206.99 0.050 1247.59 1.8662 14.4188
0.40 214.09 0.056 1551.73 2.5554 17.3438
0.50 219.52 0.061 1855.02 3.2369 19.7474
0.60 223.82 0.066 2157.63 3.9126 21.7577
0.70 227.33 0.071 2459.71 4.5840 23.4637
0.80 230.25 0.076 2761.36 5.2518 24.9298
0.90 232.72 0.081 3062.64 5.9169 26.2033
1.00 234.85 0.085 3363.63 6.5796 27.3197
================================================================
A.9.5 Psychrometric Properties of Moist Air at 10,000 kPa
Dry Bulb temperature of 320C
================================================================
W Twb v h s RH
kgw/kg_da C m3/kgda kJ/kgda kJ/kgda/K %
----------------------------------------------------------------
0.00 176.72 0.018 326.51 -0.5397 0.0000
0.05 195.85 0.019 480.31 -0.1514 6.5945
0.10 209.00 0.020 632.70 0.2054 12.2755
0.15 218.85 0.022 783.90 0.5507 17.2206
0.20 226.63 0.023 934.12 0.8889 21.5640
0.25 233.00 0.024 1083.47 1.2220 25.4093
0.30 238.33 0.025 1232.08 1.5512 28.8376
0.40 246.84 0.028 1527.40 2.2005 34.6877
0.50 253.40 0.030 1820.61 2.8409 39.4949
0.60 258.63 0.032 2112.08 3.4747 43.5153
0.70 262.94 0.034 2402.10 4.1033 46.9275
0.80 266.55 0.036 2690.88 4.7277 49.8597
0.90 269.63 0.039 2978.59 5.3487 52.4066
1.00 272.29 0.041 3265.36 5.9667 54.6395
================================================================
Pure fluid Virial Coefficients
------------------------------
T Baa Caaa Bww Cwww
C b'm^3/mol' b'm^6/mol^2' b'm^3/mol\x002' b'm^6/mol^2'
-60.0 -3.3064504913e-05 2.1778728776e-09 -1.1174019230e-02 -1.5162998768e-04
-50.0 -2.8932056455e-05 2.1163810315e-09 -7.8721344601e-03 -8.7876439931e-05
-40.0 -2.5223205510e-05 2.0616570944e-09 -5.7127237936e-03 -5.5471167065e-05
-30.0 -2.1877241883e-05 2.0127116499e-09 -4.2586206439e-03 -3.6054467433e-05
-20.0 -1.8844568169e-05 1.9687328800e-09 -3.2532396168e-03 -2.3880058317e-05
-10.0 -1.6084254149e-05 1.9290492160e-09 -2.5411800904e-03 -1.6072254169e-05
0.0 -1.3562212432e-05 1.8931009119e-09 -2.0256198165e-03 -1.0976416841e-05
10.0 -1.1249818308e-05 1.8604181700e-09 -1.6446680868e-03 -7.5982156425e-06
20.0 -9.1228522265e-06 1.8306041394e-09 -1.3578320706e-03 -5.3262047265e-06
30.0 -7.1606799362e-06 1.8033215779e-09 -1.1380508933e-03 -3.7775456074e-06
40.0 -5.3456100212e-06 1.7782822977e-09 -9.6688526113e-04 -2.7086426379e-06
50.0 -3.6623854498e-06 1.7552387450e-09 -8.3154379347e-04 -1.9621726463e-06
60.0 -2.0977774966e-06 1.7339772333e-09 -7.2300490095e-04 -1.4351072804e-06
70.0 -6.4025867871e-07 1.7143124658e-09 -6.3480699108e-04 -1.0590893108e-06
80.0 7.2026273739e-07 1.6960830728e-09 -5.6225490863e-04 -7.8820574569e-07
90.0 1.9926598215e-06 1.6791479533e-09 -5.0189060427e-04 -5.9126044774e-07
100.0 3.1847656914e-06 1.6633832580e-09 -4.5113452236e-04 -4.4682462927e-07
110.0 4.3035215681e-06 1.6486798883e-09 -4.0803910950e-04 -3.4002636972e-07
120.0 5.3551001609e-06 1.6349414114e-09 -3.7111708564e-04 -2.6044318239e-07
130.0 6.3450090665e-06 1.6220823143e-09 -3.3922027793e-04 -2.0070296438e-07
140.0 7.2781778723e-06 1.6100265349e-09 -3.1145310612e-04 -1.5554524200e-07
150.0 8.1590318924e-06 1.5987062200e-09 -2.8711011151e-04 -1.2118485357e-07
160.0 8.9915548780e-06 1.5880606719e-09 -2.6563036496e-04 -9.4876457701e-08
170.0 9.7793425843e-06 1.5780354497e-09 -2.4656385529e-04 -7.4613740198e-08
180.0 1.0525648716e-05 1.5685816023e-09 -2.2954647025e-04 -5.8919833627e-08
190.0 1.1233424489e-05 1.5596550080e-09 -2.1428120144e-04 -4.6700067957e-08
200.0 1.1905352827e-05 1.5512158075e-09 -2.0052390022e-04 -3.7137687457e-08
T Baw Caaw Caww
C b'm^3/mol\x002' b'm^6/mol^2' b'm^6/mol^2'
-60.0 -6.8305808721e-05 1.0273000716e-09 -1.8214316825e-06
-50.0 -6.1680233064e-05 1.0001595421e-09 -1.1787612409e-06
-40.0 -5.5836203092e-05 9.7107903308e-10 -7.9593677251e-07
-30.0 -5.0645881561e-05 9.4180678583e-10 -5.5678343751e-07
-20.0 -4.6007498746e-05 9.1337025409e-10 -4.0128618357e-07
-10.0 -4.1839118849e-05 8.8634392341e-10 -2.9668474376e-07
0.0 -3.8074090909e-05 8.6101819497e-10 -2.2423408862e-07
10.0 -3.4657682115e-05 8.3750672364e-10 -1.7276396504e-07
20.0 -3.1544553729e-05 8.1581500536e-10 -1.3537862024e-07
30.0 -2.8696845981e-05 7.9588431449e-10 -1.0768721224e-07
40.0 -2.6082708793e-05 7.7761982700e-10 -8.6816421215e-08
50.0 -2.3675162869e-05 7.6090853025e-10 -7.0839762898e-08
60.0 -2.1451208360e-05 7.4563050528e-10 -5.8437245597e-08
70.0 -1.9391120996e-05 7.3166589756e-10 -4.8686625860e-08
80.0 -1.7477891584e-05 7.1889908286e-10 -4.0932107713e-08
90.0 -1.5696776156e-05 7.0722101405e-10 -3.4699849863e-08
100.0 -1.4034932249e-05 6.9653039669e-10 -2.9642457363e-08
110.0 -1.2481122776e-05 6.8673412025e-10 -2.5501820730e-08
120.0 -1.1025473368e-05 6.7774722643e-10 -2.2083805133e-08
130.0 -9.6592722783e-06 6.6949259959e-10 -1.9240735645e-08
140.0 -8.3748044505e-06 6.6190050073e-10 -1.6859099163e-08
150.0 -7.1652131416e-06 6.5490802360e-10 -1.4850792059e-08
160.0 -6.0243839304e-06 6.4845852337e-10 -1.3146812982e-08
170.0 -4.9468470096e-06 6.4250104926e-10 -1.1692664630e-08
180.0 -3.9276944932e-06 6.3698980018e-10 -1.0444965002e-08
190.0 -2.9625101219e-06 6.3188361380e-10 -9.3689246298e-09
200.0 -2.0473092535e-06 6.2714549461e-10 -8.4364506623e-09
Pure fluid Virial Coefficients Derivatives
------------------------------------------
T dBaa dCaaa dBww dCwww
C b'm^3/mol\x002' b'm^6/mol^2' b'm^3/mol\x002' b'm^6/mol^2'
-60.0 4.3678901718e-07 -6.5259421081e-12 4.0907134267e-04 9.7890225556e-06
-50.0 3.9094567047e-07 -5.7925951449e-12 2.6368394754e-04 4.2599502143e-06
-40.0 3.5183089770e-07 -5.1686009316e-12 1.7524578197e-04 2.4534392599e-06
-30.0 3.1818443574e-07 -4.6339568007e-12 1.1972698408e-04 1.5168064804e-06
-20.0 2.8902947780e-07 -4.1729431726e-12 8.3872099442e-05 9.6398589135e-07
-10.0 2.6359917737e-07 -3.7730826593e-12 6.0116039515e-05 6.2432365587e-07
0.0 2.4128450184e-07 -3.4243800668e-12 4.4005975878e-05 4.1098205932e-07
10.0 2.2159662973e-07 -3.1187604739e-12 3.2846510930e-05 2.7460401649e-07
20.0 2.0413943007e-07 -2.8496489141e-12 2.4963984884e-05 1.8603615349e-07
30.0 1.8858904489e-07 -2.6116525842e-12 1.9294782853e-05 1.2767075965e-07
40.0 1.7467855065e-07 -2.4003181623e-12 1.5148499662e-05 8.8680535853e-08
50.0 1.6218630137e-07 -2.2119447569e-12 1.2068194612e-05 6.2299015990e-08
60.0 1.5092697458e-07 -2.0434384795e-12 9.7459891450e-06 4.4233623231e-08
70.0 1.4074462510e-07 -1.8921984596e-12 7.9709845791e-06 3.1722699778e-08
80.0 1.3150724657e-07 -1.7560268233e-12 6.5964818172e-06 2.2965936560e-08
90.0 1.2310247686e-07 -1.6330570877e-12 5.5189694206e-06 1.6775064043e-08
100.0 1.1543417961e-07 -1.5216968216e-12 4.6644255474e-06 1.2356543007e-08
110.0 1.0841970276e-07 -1.4205814367e-12 3.9792467564e-06 9.1745422056e-09
120.0 1.0198766459e-07 -1.3285367274e-12 3.4241524938e-06 6.8634353500e-09
130.0 9.6076153981e-08 -1.2445483295e-12 2.9700329278e-06 5.1712456914e-09
140.0 9.0631258338e-08 -1.1677366865e-12 2.5950842306e-06 3.9226742483e-09
150.0 8.5605852607e-08 -1.0973364250e-12 2.2828082790e-06 2.9946678537e-09
160.0 8.0958597486e-08 -1.0326792823e-12 2.0206000698e-06 2.3001072716e-09
170.0 7.6653106484e-08 -9.7317990685e-13 1.7987394663e-06 1.7768097568e-09
180.0 7.2657249944e-08 -9.1832399823e-13 1.6096642209e-06 1.3800452917e-09
190.0 6.8942570813e-08 -8.6765835911e-13 1.4474407409e-06 1.0773984832e-09
200.0 6.5483792067e-08 -8.2078251748e-13 1.3073752610e-06 8.4521088732e-10
T dBaw dCaaw dCaww
C b'm^3/mol\x002' b'm^6/mol^2' b'm^6/mol^2'
-60.0 7.0671067841e-07 -2.5329306643e-12 8.3652108680e-08
-50.0 6.2109405080e-07 -2.8479923244e-12 4.8634111869e-08
-40.0 5.4982837510e-07 -2.9396633262e-12 2.9766967562e-08
-30.0 4.8992187794e-07 -2.8980941059e-12 1.9020412856e-08
-20.0 4.3911281598e-07 -2.7799104397e-12 1.2604799172e-08
-10.0 3.9566848048e-07 -2.6206893674e-12 8.6179394263e-09
0.0 3.5824516845e-07 -2.4426731561e-12 6.0532052853e-09
10.0 3.2578908214e-07 -2.2596007123e-12 4.3529611359e-09
20.0 2.9746516934e-07 -2.0797672895e-12 3.1957227862e-09
30.0 2.7260533603e-07 -1.9079803096e-12 2.3895374649e-09
40.0 2.5067028507e-07 -1.7468190020e-12 1.8161835419e-09
50.0 2.3122106772e-07 -1.5974502672e-12 1.4008122071e-09
60.0 2.1389764536e-07 -1.4601590182e-12 1.0948500459e-09
70.0 1.9840257004e-07 -1.3346933582e-12 8.6606704976e-10
80.0 1.8448844334e-07 -1.2204888941e-12 6.9264384038e-10
90.0 1.7194819305e-07 -1.1168137618e-12 5.5953719449e-10
100.0 1.6060747132e-07 -1.0228614572e-12 4.5620187444e-10
110.0 1.5031866448e-07 -9.3780925812e-13 3.7513243420e-10
120.0 1.4095613794e-07 -8.6085397191e-13 3.1091182225e-10
130.0 1.3241243488e-07 -7.9123279439e-13 2.5957962745e-10
140.0 1.2459521736e-07 -7.2823445525e-13 2.1820572123e-10
150.0 1.1742478936e-07 -6.7120410110e-13 1.8459817415e-10
160.0 1.1083207914e-07 -6.1954421544e-13 1.5710036152e-10
170.0 1.0475698647e-07 -5.7271310484e-13 1.3444819325e-10
180.0 9.9147021510e-08 -5.3022196394e-13 1.1566843627e-10
190.0 9.3956178059e-08 -4.9163118437e-13 1.0000548446e-10
200.0 8.9143996459e-08 -4.5654633851e-13 8.6868060073e-11
Water saturation pressure p_ws [kPa]
T p_ws
C b'Pa\x00/mol^2'
-60.00 1.0813475449e+00
-30.00 3.8005139487e+01
0.00 6.1115347506e+02
30.00 4.2466883405e+03
60.00 1.9945801925e+04
90.00 7.0182360745e+04
120.00 1.9866539974e+05
150.00 4.7610138108e+05
180.00 1.0026345688e+06
210.00 1.9073906643e+06
240.00 3.3466518715e+06
270.00 5.5028394741e+06
300.00 8.5877083296e+06
Henry Constant (zero for T < 273.15 K)
T beta_H
C b'1/Pa\x00ol^2'
0.01 2.2594633839e-10
30.01 1.3058555542e-10
60.01 1.0117905765e-10
90.01 9.5497073897e-11
120.01 1.0310894778e-10
150.01 1.2209642969e-10
180.01 1.5416532918e-10
210.01 2.0384427379e-10
240.01 2.7939520108e-10
270.01 3.9586839028e-10
300.01 5.8396949465e-10
Isothermal Compressibility of water (kT) [1/Pa]
T p = 101325.000 Pa p = 200000.000 Pa p = 500000.000 Pa p = 1000000.000 Pa
-60.00 1.0771099108e-10 1.0770400843e-10 1.0768278304e-10 1.0764742021e-10
-30.00 1.1257575753e-10 1.1256891351e-10 1.1254810951e-10 1.1251344878e-10
0.00 1.1778484390e-10 1.1777815515e-10 1.1775782318e-10 1.1772394894e-10
30.00 1.0048218396e-03 1.0047775852e-03 1.0046431164e-03 1.0044192594e-03
60.00 1.0173376853e-03 1.0172931915e-03 1.0171579998e-03 1.0169329553e-03
90.00 1.0360937793e-03 1.0360454263e-03 1.0358985238e-03 1.0356540343e-03
120.00 1.7695003917e+00 1.0604394263e-03 1.0602707553e-03 1.0599901229e-03
150.00 1.9111838106e+00 9.5989413316e-01 1.0905701909e-03 1.0902325237e-03
180.00 2.0511819911e+00 1.0326461304e+00 4.0465521554e-01 1.9441817992e-01
210.00 2.1901914433e+00 1.1043221088e+00 4.3506032354e-01 2.1154168024e-01
240.00 2.3285841090e+00 1.1753398746e+00 4.6467604880e-01 2.2755082652e-01
270.00 2.4665706339e+00 1.2459309799e+00 4.9380122272e-01 2.4295534461e-01
300.00 2.6042765544e+00 1.3162310320e+00 5.2260279146e-01 2.5797921396e-01
Molar volume of saturated liquid water or ice (vbar_ws) [m^3/mol_H2O]
T p = 101325.000 Pa p = 200000.000 Pa p = 500000.000 Pa p = 1000000.000 Pa
-60.00 1.9483157215e-11 1.9482950148e-11 1.9482320703e-11 1.9481271948e-11
-30.00 1.9562306493e-11 1.9562089195e-11 1.9561428642e-11 1.9560328042e-11
0.00 1.9651836970e-11 1.9651608576e-11 1.9650914289e-11 1.9649757465e-11
30.00 1.8094773222e-05 1.8094773222e-05 1.8094773222e-05 1.8094773222e-05
60.00 1.8323837443e-05 1.8323837443e-05 1.8323837443e-05 1.8323837443e-05
90.00 1.8662959891e-05 1.8662959891e-05 1.8662959891e-05 1.8662959891e-05
120.00 1.9102048132e-05 1.9102048132e-05 1.9102048132e-05 1.9102048132e-05
150.00 1.9645709876e-05 1.9645709876e-05 1.9645709876e-05 1.9645709876e-05
180.00 2.0310359748e-05 2.0310359748e-05 2.0310359748e-05 2.0310359748e-05
210.00 2.1126885602e-05 2.1126885602e-05 2.1126885602e-05 2.1126885602e-05
240.00 2.2149039824e-05 2.2149039824e-05 2.2149039824e-05 2.2149039824e-05
270.00 2.3473849596e-05 2.3473849596e-05 2.3473849596e-05 2.3473849596e-05
300.00 2.5297523418e-05 2.5297523418e-05 2.5297523418e-05 2.5297523418e-05
Enhancement factor (f) [no units]
T p = 101325.000 Pa p = 200000.000 Pa p = 500000.000 Pa p = 1000000.000 Pa p = 10000000.000 Pa
-60.00 1.0070775889e+00 1.0140339780e+00 1.0356182628e+00 1.0730973444e+00 2.2389383691e+00
-40.00 1.0056000404e+00 1.0110608386e+00 1.0279266142e+00 1.0569409210e+00 1.8450348547e+00
-20.00 1.0046363568e+00 1.0090315492e+00 1.0225621564e+00 1.0456875555e+00 1.6193678937e+00
0.00 1.0041972674e+00 1.0078137836e+00 1.0189177377e+00 1.0378059886e+00 1.4778432214e+00
40.00 1.0048337245e+00 1.0074421047e+00 1.0151963013e+00 1.0282275373e+00 1.3082436191e+00
80.00 1.0057272574e+00 1.0097059521e+00 1.0168897805e+00 1.0272924735e+00 1.2343415184e+00
120.00 1.0000000000e+00 1.0001669826e+00 1.0183856131e+00 1.0312270756e+00 1.2048250858e+00
160.00 1.0000000000e+00 1.0000000000e+00 1.0000000000e+00 1.0231647493e+00 1.2031653250e+00
200.00 1.0000000000e+00 1.0000000000e+00 1.0000000000e+00 1.0000000000e+00 1.2128825018e+00
250.00 1.0000000000e+00 1.0000000000e+00 1.0000000000e+00 1.0000000000e+00 1.1903236875e+00
300.00 1.0000000000e+00 1.0000000000e+00 1.0000000000e+00 1.0000000000e+00 1.0480338998e+00
350.00 1.0000000000e+00 1.0000000000e+00 1.0000000000e+00 1.0000000000e+00 1.0000000000e+00
Verification Script¶
This script, written in Python, should yield no failures:
import CoolProp.CoolProp as CP
import numpy as np
import itertools
from multiprocessing import Pool
CP.set_config_bool(CP.DONT_CHECK_PROPERTY_LIMITS, True)
def generate_values(TR,P=101325):
""" Starting with T,R as inputs, generate all other values """
T,R = TR
psi_w = CP.HAPropsSI('psi_w','T',T,'R',R,'P',P)
other_output_keys = ['T_wb','T_dp','Hda','Sda','Vda','Omega']
outputs = {'psi_w':psi_w,'T':T,'P':P,'R':R}
for k in other_output_keys:
outputs[k] = CP.HAPropsSI(k,'T',T,'R',R,'P',P)
return outputs
def get_supported_input_pairs():
""" Determine which input pairs are supported """
good_ones = []
inputs = generate_values((300, 0.5))
for k1, k2 in itertools.product(inputs.keys(), inputs.keys()):
if 'P' in [k1,k2] or k1==k2:
continue
args = ('psi_w', k1, inputs[k1], k2, inputs[k2], 'P', inputs['P'])
try:
psi_w_new = CP.HAPropsSI(*args)
if not np.isfinite(psi_w_new):
raise ValueError('Returned NaN; not ok')
good_ones.append((k1,k2))
except BaseException as BE:
pass
if 'currently at least one of' in str(BE) or 'cannot provide two inputs' in str(BE):
pass
else:
print(BE)
good_ones.append((k1,k2))
return good_ones
supported_pairs = get_supported_input_pairs()
def calculate(inputs):
""" For a given input, try all possible input pairs """
errors = []
for k1, k2 in supported_pairs:
psi_w_input = inputs['psi_w']
args = 'psi_w',k1,inputs[k1],k2,inputs[k2],'P',inputs['P']
try:
psi_w_new = CP.HAPropsSI(*args)
if not np.isfinite(psi_w_new):
raise ValueError('Returned NaN; not ok')
except BaseException as BE:
errors.append((str(BE),args, inputs))
return errors
if __name__ == '__main__':
import CoolProp
print(CoolProp.__version__)
TR = itertools.product(np.linspace(240, 345, 11), np.linspace(0, 1, 11))
with Pool(processes=2) as pool:
input_values = pool.map(generate_values, TR)
errors = pool.map(calculate, input_values)
for err in itertools.chain.from_iterable(errors):
print(err)

